What is Inside a Battery

What is inside a battery?
You’ll get a real charge out of the answer.
What are the parts of a battery?
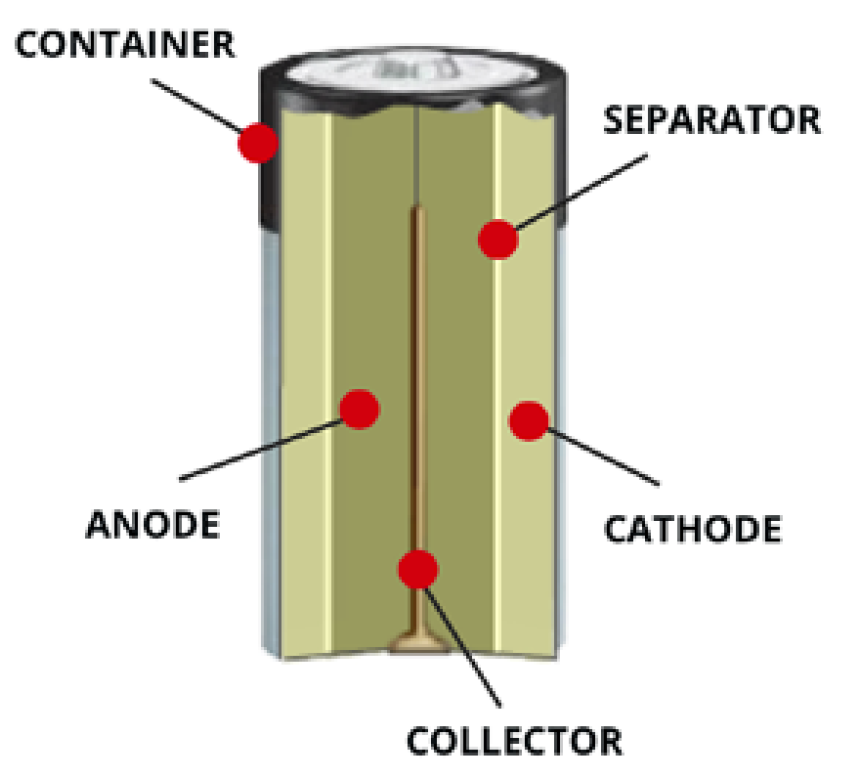
Parts of a battery
The answer to “what is inside a battery?” starts with a breakdown of what makes a battery a battery.
Container Steel can that houses the cell’s ingredients to form the cathode, a part of the electrochemical reaction.
Cathode A combo of manganese dioxide and carbon, cathodes are the electrodes reduced by the electrochemical reaction.
Separator Non-woven, fibrous fabric that separates the electrodes.
Anode Made of powered zinc metal, anodes are electrodes that are oxidized.
Electrolyte Potassium hydroxide solution in water, the electrolyte is the medium for the movement of ions within the cell. It carries the ionic current inside the battery.
Collector Brass pin in the middle of the cell that conducts electricity to the outside circuit.