How Do Batteries Work?

How do batteries work?
It’s not exactly magic … but it’s close.
Think of a battery as a small power plant that converts a chemical reaction into electrical energy. Various dry cell (or alkaline) batteries can differ in several ways, but they all have the same basic components. For even more details, visit our What’s Inside a Battery page or our Battery Chemistry page.
Powering the device
Let’s look at how batteries work to generate and send power to your favorite devices. More technical details can be found on our Battery Chemistry page
Powering the device
The chemical reaction starts when you insert a battery into a device – and complete the circuit.
Device Responds
The electrolyte oxides the anode’s powered zinc. The cathode’s manganese dioxide/carbon mix reacts with the oxidized zinc to produce electricity.
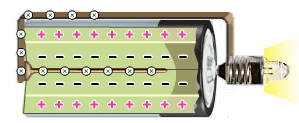
Voltage Drops
Interaction between the zinc and the electrolyte produces reaction products, which gradually slows the cell’s action and lowers its voltage.
Constructing the battery
It’s easier to understand how batteries work when you see how they’re put together.
Container —It all starts with an empty steel can – the battery container.
Cathode Mix —Finely-ground powders of manganese dioxide and conductors that carry a naturally-occurring electrical charge are molded to the inside wall of the empty container.
Separator —Separator paper is inserted to keep the cathode from touching the anode.
Anode —The anode, which carries a negative electrical charge, plus potassium hydroxide electrolyte are then pumped into each container.
Collector —The brass pin, which forms the negative current collector, is inserted into the battery, which is then sealed and capped.